Abstract
Background: Therapy-related acute myeloid leukemia (t-AML) represents emerging challenge of the modern oncology as a life-threatening complication of cytotoxic therapy. Disease characterises poor prognosis and presence of adverse cytogenetic and genetic abnormalities.
The goal of the study: Clinical outcome of t-AML patients with respect to genetic changes and treatment intensity.
Patients and methods: Retrospective analysis of all consecutive AML patients treated in years 2000-2021 in one hematological center was performed. Diagnosis of t-AML was established according to WHO 2016 criteria. Overall survival (OS) and progression free survival (PFS) was defined to evaluate treatment outcomes only within t-AML patients undergoing intensive treatment (standard induction/consolidation; allogeneic cell transplantation (alloHCT) if eligible).
Results: Among 743 AML patients 60 (8.1%) were diagnosed as t-AML (38 woman) with median age 57 years. Solid tumors (ST) preceded t-AML in 63.3%, hematological neoplasms (HN) in 36.7%. Majority of t-AML was preceded by breast cancer (30.0%), Hodgkin Lymphoma (11.7%), non-Hodgkin Lymphoma (10.0%) and ovarian cancer (10.0%). Median latency time for ST and HN subgroups was 5 vs 7 years respectively (P = .036). Previous cytotoxic therapy consisted of chemotherapy, radiotherapy or combination in 56.6%, 18.3% and 25.0% (autologous cell transplantation was performed in 54.5% of HN).
Cytogenetic and molecular biology analysis was performed in 44 and 27 of t-AML respectively. Cytogenetic abnormalities, complex karyotype and normal karyotype occurred in 78.9%, 28.9% and 15.8% patients. KMT2A, RUNX1-RUNX1T1 and PML-RARA rearrangement was found in 21.1%, 18.4% and 7.9% of t-AML. FLT3-ITD, FLT-TKD, NPM1 and C-KIT DNA sequence variant occurred as follows: 14.8%, 7.4%, 3.7% and 3.7% correspondingly. Three pathogenic TP53 DNA sequence variants were detected in t-AML patients: c.711G>A, c.704A>G and c.989T>C (analysis performed on 20 t-AML patients). According ELN2017 genetic risk stratification patients were classified as adverse, intermediate and favorable in 51.4%, 35.1% and 13.5% respectively.
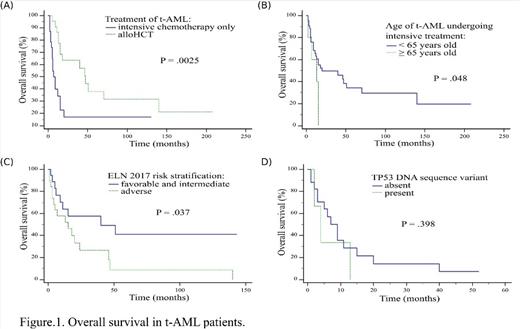
Intensive treatment was implemented in 48 patients including alloHCT in 23 of them. Median OS and PFS was 15 and 8 months respectively for whole treated group. Median OS in t-AML undergoing intensive chemotherapy only vs alloHCT was 7 vs 47 months (P = .0025) with 12-year OS after alloHCT- 21.1% (Fig.1A). Among therapy-related acute promyelocytic leukemia (t-APL) patients median OS was not reached, without alloHCT. Median OS was higher for t-AML patients younger than 65 years than older ones: 20 vs 13 months respectively (P = .048) (Fig.1B). Among t-AML median OS in subgroup with adverse ELN 2017 vs intermediate and favorable ELN 2017 was 15 vs 40 months (P = .037) with 5-years OS 8.2% vs 41.0% (Fig.1C). In multivariate Cox proportional hazard regression model alloHCT was the only factor significantly influencing OS (HR = 0.16, 95% CI = 0.05-0.56, P = .004).
All patients with TP53 mutations were intensively treated, one patient underwent alloHCT. 66.6% of patients had complex karyotype and any co-occuring DNA sequence variant was detected. Importantly, c.704A>G and c.989T>C TP53 DNA sequence variants were not previously described in AML according Catalogue of Somatic Mutations In Cancer database. Median OS in t-AML with TP53 mutation vs without was 4 vs 7 months (P = .398) (Fig.1D).
Conslusions: Our study brings detailed analysis of clinical outcome of t-AML. Patients with t-AML undergoing intensive treatment, younger than 65 years and with t-APL have significantly higher OS rates. On the contrary t-AML patients classified as adverse genetic ELN2017 subgroup have poorer OS rates. Treatment strategy in t-AML should rely on performing alloHCT possibly soon.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal